(i) Isothermal compression of 1 mole of an ideal gas at 273K from
1 to 2atm pressure. (1atm=1.013![]() Pa.)
Pa.)
See here for a similar example.
(ii) Adiabatic compression of 1 mole of an ideal gas
initially at 273K from 1 to 2atm pressure. (Take
![]() ).
).
Adiabatic compression is covered in this example.
(iii) Isothermal compression of 1 mole of water at 273K from
1 to 2atm pressure. The density of
water is 1000kgm![]() . The bulk modulus
. The bulk modulus ![]() of water (at 273K and in
the pressure range indicated) is
of water (at 273K and in
the pressure range indicated) is
![]() Nm
Nm![]() . The bulk
modulus is the elastic modulus that relates the volume change
. The bulk
modulus is the elastic modulus that relates the volume change ![]() to
the pressure change
to
the pressure change ![]() that produces it by
that produces it by
![]() , where
, where ![]() is the initial volume.
is the initial volume.
Remember, water is not an ideal gas! We can't use PV=nRT!
Instead use the information in the question to write the ![]() of
of ![]() in terms of
in terms of ![]() .
.
(iv) Isothermal magnetization of 1 mole of a paramagnetic sample at
273K from an external field of 1T to an external field of 2T.
Assume that the equation of state is
with
A model paramagnet is described here; we will meet it again later in the course.
(v) Isothermal stretching of an elastic string from 0.1m to 0.2m at
273K. The equation of state linking the tension ![]() to the length
to the length ![]() of the string is
of the string is
where ![]() NK
NK![]() and
and ![]() m is the unstretched length.
m is the unstretched length.
The work done in stretching a string is given here - but
try to guess it first.
In cases (i), (ii) and (iv), how much heat is absorbed or given out by the
system? You may assume that the internal energy of the paramagnet is ![]() .
.
Here we use the first law, so we can only find the answer
for those cases where we know the internal energy - as we do for an ideal gas
and an ideal paramagnet.
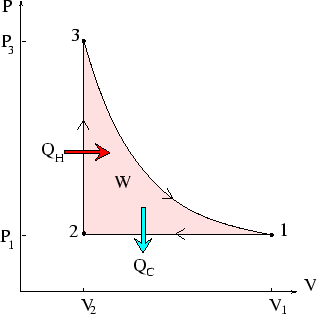
A quantity of ideal gas is taken through the reversible cycle depicted above, where
the three processes are isobaric, isochoric
and adiabatic respectively. Calculate the
work done on the system, and the heat absorbed, during each stage, and check that
the total work done by the system is equal to the net heat absorbed--which
equals
![]() .
.
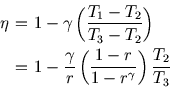
where
The Otto cycle is covered here. Only the isobaric stroke is
new.
This is lower than that of a Carnot engine operating between
the lowest and highest temperatures attained in the cycle (![]() and
and ![]() respectively).
This is hard to prove algebraically, but using your graphical calculator you should
be able to convince yourself that the function of
respectively).
This is hard to prove algebraically, but using your graphical calculator you should
be able to convince yourself that the function of ![]() multiplying
multiplying ![]() is greater than 1
for all positive
is greater than 1
for all positive ![]() less than 1 (with
less than 1 (with ![]() ).
).
It's much easier to prove for the Otto cycle. Try it!