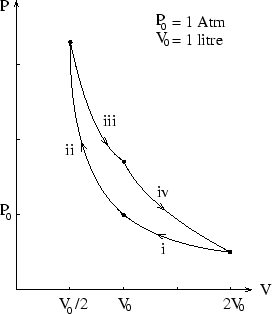

In this cycle the system, which is an ideal monatomic gas, undergoes first isothermal (i) then adiabatic (ii)
compression steps, each of which halve the volume, then returns to its starting point via first isothermal (iii)
and then adiabatic (iv) expansions, each of which double the volume. How much work is done by the system during
the cycle if ![]() is 1 atmosphere and
is 1 atmosphere and ![]() litre?
litre?
The short answer is 41.3 J. The full answer is here.