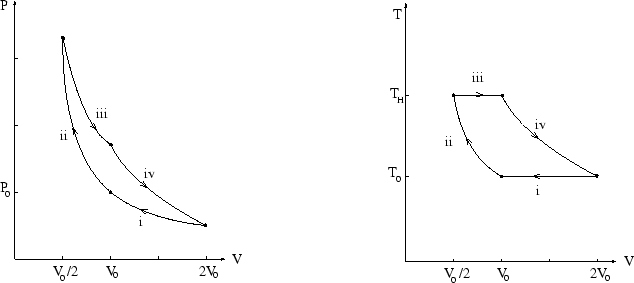

We have already calculated the work done on the gas during an isothermal volume change:
For the adiabatic stages we use ![]() (see properties of an ideal gas) to
get
(see properties of an ideal gas) to
get
(Note that ![]() !
Can you think of an easier way of obtaining this expression for the work?
Hint: no heat is exchanged, so
!
Can you think of an easier way of obtaining this expression for the work?
Hint: no heat is exchanged, so ![]() .)
.)
All we need is the temperature of the isothermal expansion, which is
also the temperature at the end of the adiabatic compression. Using ![]() gives
gives ![]() so
so ![]() where
where ![]() .
.
So
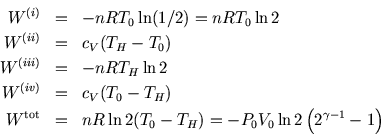
The work done during the adiabatic steps (ii) and (iv) cancels. We could
have guessed this, as the work done by the system during the cycle must
equal the net heat absorbed. Heat is only absorbed or given out in the
isothermal steps, and its magnitude is equal and opposite to the work done
on the system in those steps. So we could more simply have written ![]() .
.