1.) First year revision quiz
Parts 1-5 test the first law, work done on a fluid and properties of an ideal gas. Not all of these will have been met in lectures this year by the time you do this sheet, but they were all covered in first year. As a hint for parts 2 and 3, remember that during a reversible adiabatic process entropy is constant.
Useful links for this quiz are the first law of thermodynamics, the properties of an ideal gas, work (and worked example).
1.) In some process a fluid starts at ![]() ,
, ![]() ,
, ![]() , and ends at
, and ends at ![]() ,
, ![]() ,
, ![]() . Which of the following
are true?
. Which of the following
are true?
2.) An ideal monatomic gas is slowly taken from ![]() ,
, ![]() ,
, ![]() , to
, to ![]() ,
, ![]() ,
, ![]() .
Match the work done on the gas with the conditions.
.
Match the work done on the gas with the conditions.
| a.) Constant pressure | i)
|
| b.) Constant volume | ii)
|
| c.) Constant temperature | iii)
|
| d.) Constant entropy | iv) 0 |
3.)
Match the following expressions for the heat transferred to the system during the process described in
question 2 with the conditions.
| a.) Constant pressure | |
| b.) Constant volume | |
| c.) Constant temperature | |
| d.) Constant entropy |
4.) How well do the following substances approximate to an ideal gas? Rank on a scale of 0 (not at all) to 3 (very well indeed).
5.) Which of the following applies to all fluids, and which to ideal gases only?
6.)
You have a number of heat reservoirs available, the hottest being at ![]() and the coolest being at
and the coolest being at ![]() .
Which engine (a-d) will you choose for maximum efficiency?
.
Which engine (a-d) will you choose for maximum efficiency?
| a.) Diesel |
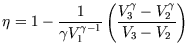 |
| b.) Joule ideal gas |
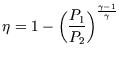 |
| c.) Carnot |
|
| d.) Otto |
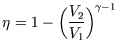 |
The maximum score is 26. Any mark above 21 is very good. Anything less requires some revision! See the following links: ideal gas, the first law, work, heat engines.