




Next: Ideal Gas: Recap
Previous: 5. Systems with variable particle number
Glossary
- Adiabatic Of processes: occurring without heat transfer. Of walls:
insulating. Reversible adiabatic processes are isentropic.
- Equation of State A relationship between the state variables of the system.
Simple
systems in equilibrium are fully specified by two properties such as temperature and volume; all the other
functions of state are functions of these. For an ideal gas, the equation of state is
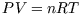 ; for an ideal
paramagnet it is
; for an ideal
paramagnet it is 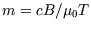 where
where 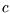 is a constant.
is a constant.
- Equilibrium A system is in equilibrium when its macroscopic properties
(temperature, pressure) are uniform and not changing with time.
- Extensive Some thermodynamic functions of state are extensive: if the
intensive variables (
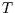 ,
, 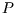 ,
, 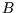 ) are kept constant, the extensive variables are proportional to
the amount of substance present. Energy, volume, entropy, total magnetisation, enthalpy, Gibbs and Helmholtz free
energies and heat capacities are all extensive. Extensive properties expressed per unit mass or per mole
are then intensive, and are called specific: eg. specific heat capacity. All functions of state are
either extensive or intensive.
) are kept constant, the extensive variables are proportional to
the amount of substance present. Energy, volume, entropy, total magnetisation, enthalpy, Gibbs and Helmholtz free
energies and heat capacities are all extensive. Extensive properties expressed per unit mass or per mole
are then intensive, and are called specific: eg. specific heat capacity. All functions of state are
either extensive or intensive.
- Function of state A property of a system which only depends of the current state
of the system and not on its history. Examples are temperature, pressure, volume, internal energy, entropy,
magnetisation for a paramagnet (but not for a ferromagnet). Also called a state variable or a
macroscopic variable.
- Heat Reservoir Surroundings at a certain temperature which are large
enough to absorb heat from, or donate heat to, the system without appreciable change of temperature. Also
heat bath
- Intensive An intensive functions of state does not vary
with the amount of the substance present. Examples are temperature, pressure, tension and external magnetic field.
All functions of state are either extensive or intensive.
- Isentropic At constant entropy.
- Isobaric At constant pressure.
- Isochoric At constant volume.
- Isothermal At constant temperature.
- Macroscopic Pertaining to aspects of a system which can be measured
classically, such as temperature, pressure etc; containing very large numbers of atoms.
- Macrostate The current disposition of the system defined in
terms of macroscopic variables. Also (in classical thermal physics) state.
- Microscopic Pertaining to the underlying quantum state of a system
- Microstate The state of the system defined in terms of the current behaviour
of all the constituent atoms. NOT ``the state of a very small system''! Also quantum state.
- Reversible A reversible process is one which would change direction with an
infinitesimal change in external conditions. For a process to be reversible it must be frictionless and
quasistatic so that no energy is dissipated and the system is always only infinitesimally removed from
equilibrium. Because a reversible process passes through a series of quasi-equilibrium
states it can be represented as a solid line on a plot of one state variable against another. More details
here.
- Surroundings That part of an experimental set up, including the ambient
air, which exchanges heat with, or does work on, the system.
- System That part of an experimental system in which we are primarily interested.
- Universe A deliberately grandiose term for the entire experimental set-up,
comprising the system and the surroundings. Usually encountered in the phrase ``the entropy change of the
universe'' which of course cannot be negative, though that of the system or surroundings alone may be.
It should not be confused with the cosmological universe.





Next: Ideal Gas: Recap
Previous: 5. Systems with variable particle number
Judith McGovern
2004-03-17