Similar examples can be found here.
- (a)
- For each of the processes (i), (ii) and (iv) of question 2 on examples 2, what is the entropy change of the system, and of the universe?
- (b)
- A 10kg lump of copper at
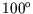 C is cooled reversibly to
C is cooled reversibly to
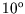 C. Calculate the change in entropy of the block. What is the
change in entropy of the universe as a result of the process?
C. Calculate the change in entropy of the block. What is the
change in entropy of the universe as a result of the process?
- (c)
- A 10kg lump of copper at
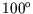 C is cooled irreversibly by
dumping it in a lake at
C is cooled irreversibly by
dumping it in a lake at 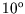 C. What is the change in entropy of the
block? What is the change in entropy of the universe?
C. What is the change in entropy of the
block? What is the change in entropy of the universe?
(The specific heat capacity of copper is 375Jkg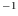 K
K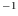 )
)
More worked examples of entropy changes are found here.
- (a)
- At atmospheric pressure, lead melts at
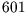 K, its density decreasing
from
K, its density decreasing
from
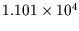 kgm
kgm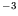 to
to
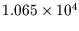 kgm
kgm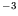 . The latent heat of
fusion is 24.5kJkg
. The latent heat of
fusion is 24.5kJkg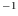 . Estimate the melting point at 100 atmospheres.
. Estimate the melting point at 100 atmospheres.
- (b)
- The melting point of ice falls with increasing pressure. Thus the
pressure of the blade of an ice skate can cause ice to melt and lubricate
the skate, allowing the skater to glide with very little friction. If the
ice temperature is too low this cannot happen and it spoils the skating.
Calculate a lowest temperature for good skating. You may assume that the
effective area of contact between the blade and the ice is 5mm
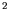 and
that the skater's mass is 60kg.
and
that the skater's mass is 60kg.
(The latent heat of fusion of ice is
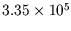 Jkg
Jkg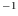 and
the densities of ice and water near 0
and
the densities of ice and water near 0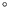 C are 0.9168gcm
C are 0.9168gcm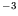 and
0.9999gcm
and
0.9999gcm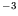 respectively).
respectively).
Can you think of any effects which you have ignored which may significantly change this estimate?
These problems use the Clausius-Clapeyron equation. Remember to refer all quantities (latent heat, volume change) to the same amount of material, and be careful in calculating the volume change from the density change.