We can apply these to the spin-
![]() paramagnet in a magnetic field.
There is just one difference: we can't derive pressure in this case, because the work is
magnetic and not mechanical.
Instead of
paramagnet in a magnetic field.
There is just one difference: we can't derive pressure in this case, because the work is
magnetic and not mechanical.
Instead of ![]() we have
we have ![]() in the fundamental thermodynamic relation, so we have an expression for
in the fundamental thermodynamic relation, so we have an expression for
![]() instead of
instead of ![]() :
:
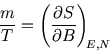
Now for an isolated system the energy is fixed, and therefore so is the number of up spins:
![]() (note
(note ![]() is now the magnetic moment!)
Then we have
is now the magnetic moment!)
Then we have
For large numbers of spins, we can use Stirling's approximation:
So
There will always be more spins aligned with the field than against it, so ![]() is positive.
is positive.
Differentiating the entropy with respect to ![]() instead, and using the above result for
instead, and using the above result for ![]() , we get an expression for
, we get an expression for
![]() :
: