Consider an isolated system of ![]() atoms in a box of volume
atoms in a box of volume ![]() . Imagine the box subdivided into many tiny cells
of volume
. Imagine the box subdivided into many tiny cells
of volume ![]() , so that there are
, so that there are ![]() cells in all (this number should be much greater than
cells in all (this number should be much greater than ![]() ).
Now each atom can be in any cell, so there are
).
Now each atom can be in any cell, so there are ![]() microstates for each atom, and
microstates for each atom, and
![]() microstates for the gas as a whole. Thus
microstates for the gas as a whole. Thus
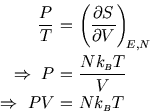
A problem with this expression for the entropy is that it depends on the size ![]() of the
imaginary cells into which we subdivided our box. This is clearly unsatisfactory
(though at least entropy changes are independent of it), but classical physics can't do any better.
Quantum physics can though! If you want to jump ahead to see the full expression
(called the Sackur-Tetrode equation) see here.
of the
imaginary cells into which we subdivided our box. This is clearly unsatisfactory
(though at least entropy changes are independent of it), but classical physics can't do any better.
Quantum physics can though! If you want to jump ahead to see the full expression
(called the Sackur-Tetrode equation) see here.