As we have seen, the fundamental thermodynamic relation
![]() implies that the natural variable in which to express
implies that the natural variable in which to express ![]() are
are ![]() and
and ![]() :
: ![]() .
.
That means that on purely mathematical grounds, we can write
But comparison with the fundamental thermodynamic relation, which contains the physics, we can make
the following identifications:
These (especially the second) are interesting in their own right. But we can go further, by differentiating
both sides of the first equation by ![]() and of the second by
and of the second by ![]() :
:
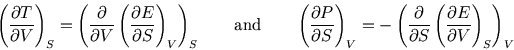
By starting with ![]() ,
, ![]() and
and ![]() , we can get three more relations.
, we can get three more relations.
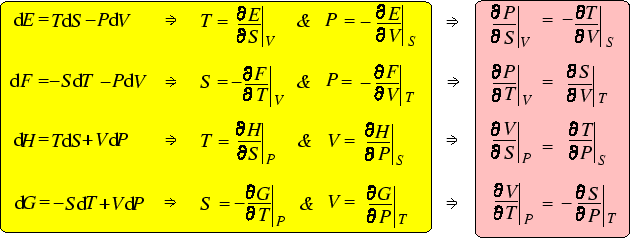
The two equations involving derivatives of ![]() are particularly useful, as they provide a handle on
are particularly useful, as they provide a handle on ![]() which
isn't easily experimentally accessible.
which
isn't easily experimentally accessible.
For non-hydrodynamic systems, we can obtain analogous relations involving, say, ![]() and
and ![]() instead of
instead of ![]() and
and ![]() ;
for instance by starting with
;
for instance by starting with
![]() we get
we get
![]() .
.
To fully exploit these relations, some properties of partial derivatives are useful. See here for a refresher course!
In maths, it's usually quite obvious what the independent variables are: either ![]() or
or
![]() ,
for instance, and if you differentiate with respect to one you know that you are keeping the others constant.
In thermal physics it isn't obvious at all, so always specify what is being held constant.
Expressions like
,
for instance, and if you differentiate with respect to one you know that you are keeping the others constant.
In thermal physics it isn't obvious at all, so always specify what is being held constant.
Expressions like
References