Let's look again at the fundamental thermodynamic relation for a hydrodynamic system:
![]() .
.
This suggests that the natural variable in which to express ![]() are
are ![]() and
and ![]() :
: ![]() .
That means that energy will be unchanged for processes at constant volume and entropy--not
the most common experimental conditions. It is useful to introduce other functions of state, called
``thermodynamic potentials'', which are conserved, or whose change is easily calculated,
in common experimental conditions.
.
That means that energy will be unchanged for processes at constant volume and entropy--not
the most common experimental conditions. It is useful to introduce other functions of state, called
``thermodynamic potentials'', which are conserved, or whose change is easily calculated,
in common experimental conditions.
These are
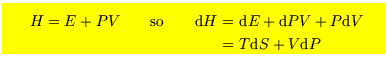
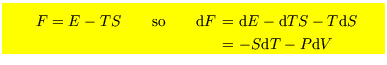
![$\mbox{\large\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle
G=E-TS+PV \qquad\hbox{so}\qquad {\rm d}G=-S{\rm d}T+V{\rm d}P $ }}$](img239.gif)
The Gibbs free energy is constant if the temperature and pressure are constant. These are the conditions under which phase transitions (melting, boiling) take place, and are also relevant to chemical equilibrium.
Note that Enthalpy and Gibbs free energy are only relevant to hydrodynamic systems for which
![]() . However the energy and the Helmholtz free energy is more general,
with
. However the energy and the Helmholtz free energy is more general,
with
![]() and
and
![]()
References