The results for vibrational, rotational and translational energies demonstrate that, at high enough temperatures,
the law of equipartition of energy
holds: each quadratic term in the classical expression for the energy contributes
![]() to
the average energy and
to
the average energy and
![]() to the heat capacity. The oscillator has quadratic kinetic and potential terms:
to the heat capacity. The oscillator has quadratic kinetic and potential terms:
Here is an idealised graph of the heat capacity of hydrogen with temperature, (©P. Eyland, University of New South Wales)
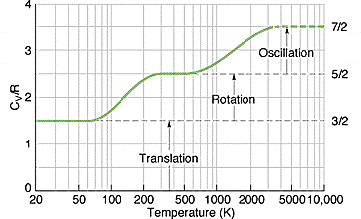
We can predict the specific heat of other substances based on equipartition, simply by counting the degrees
of freedom. For a solid, we expect the molar heat capacity to be ![]() since each atom is free to vibrate
in three directions. This is the law of Dulong and Petit, and it works well for a variety of solids at
room temperature. (More details here.)
since each atom is free to vibrate
in three directions. This is the law of Dulong and Petit, and it works well for a variety of solids at
room temperature. (More details here.)
Equipartition does not hold, even at high temperatures, if the energy is not quadratic. For instance the
gravitational potential energy is linear in height, and the average potential energy of a molecule in an
isothermal atmosphere is
![]() , not
, not
![]() .
.
Similarly the kinetic energy of a highly relativistic particle is given by the non-quadratic
![]() , not by the quadratic
, not by the quadratic
![]() , and the average kinetic
energy is
, and the average kinetic
energy is
![]() , not
, not
![]() .
.
References