If we have a mixture of two substances present, the internal energy and all the other thermodynamical potentials will depend on how much of each is present, since there will be interactions between the molecules of each.
Here we focus on the Gibbs free energy, since the relevant conditions are usually those of fixed temperature and pressure.
We have
![]() where
where ![]() and
and ![]() are the number of molecules of each substance. (This is easily
generalised more than two components.)
So
are the number of molecules of each substance. (This is easily
generalised more than two components.)
So
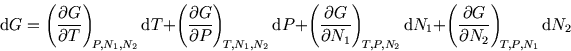
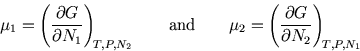
First, imagine only one substance present. Then
So for a single component system, ![]() is just the Gibbs free energy per molecule.
is just the Gibbs free energy per molecule.
But for a two component system, ![]() depends not only on the extensive variable
depends not only on the extensive variable ![]() but also
on the ratio
but also
on the ratio ![]() , which is intensive. The Gibbs free energy per molecule of one substance
can depend on the concentration of the other. All we can say is
, which is intensive. The Gibbs free energy per molecule of one substance
can depend on the concentration of the other. All we can say is ![]() is the extra Gibbs free energy
per added molecule of substance 1. If substance 1 is ethanol and substance 2 water, the chemical potential
of ethanol is different in beer (5%) and vodka (40%).
is the extra Gibbs free energy
per added molecule of substance 1. If substance 1 is ethanol and substance 2 water, the chemical potential
of ethanol is different in beer (5%) and vodka (40%).
However for ideal gases, since there are no intermolecular interactions, the Gibbs free energies are independent
and additive:
![]() .
.
From ![]() ,
, ![]() and
and ![]() (see here), we see that the term
(see here), we see that the term
![]() is added to
is added to ![]() ,
, ![]() and
and ![]() also, and
also, and
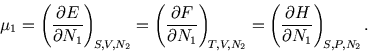
The chemical potential also governs systems which can exchange particles with a reservoir, and that is the context in which we will meet it in statistical physics.
Note: the use of ![]() to mean magnetic moment as well as chemical potential should never confuse, as magnets
tend to have fixed numbers of atoms.
to mean magnetic moment as well as chemical potential should never confuse, as magnets
tend to have fixed numbers of atoms.
If like most students you find the chemical potential mystifying, look at this helpful slide from Peter Saeta of Harvey Mudd College. The last point will only become clear once we've done Gibbs distributions at the end of the course.
References