We have already seen that for electrons in metal, the number of states with energies of order
![]() is much less that
the number of electrons to be accommodated. Because electrons are fermions, they can't occupy the same levels,
so levels up to an energy far above
is much less that
the number of electrons to be accommodated. Because electrons are fermions, they can't occupy the same levels,
so levels up to an energy far above
![]() will need to be filled. The occupancy is given by
will need to be filled. The occupancy is given by
![]() which is plotted below:
which is plotted below:
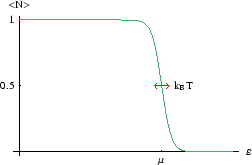
Only levels within a few
![]() of
of ![]() have occupancies which are different from 0 or 1. But what is
have occupancies which are different from 0 or 1. But what is ![]() ?
Normal metals exist in isolated chunks, and are not in contact with ``electron reservoirs''.
The answer is that we choose it so that the metal contains the right number of electrons:
?
Normal metals exist in isolated chunks, and are not in contact with ``electron reservoirs''.
The answer is that we choose it so that the metal contains the right number of electrons:
![]() . For copper at room temperature,
. For copper at room temperature, ![]() eV, whereas
eV, whereas
![]() eV
eV
The fact that thermal fluctuations affect only a small fraction of all the electrons has a number of consequences.
for instance the electronic heat capacity is much less than the
![]() predicted by equipartition.
Most of the resistance to compression of metals is due to the fact that, if the volume is reduced, the energy of all
the single-electron states increases, increasing the electronic internal energy.
predicted by equipartition.
Most of the resistance to compression of metals is due to the fact that, if the volume is reduced, the energy of all
the single-electron states increases, increasing the electronic internal energy.
References