Before reading this section, you should read over the derivation
of ![]() which held for the paramagnet,
where all
which held for the paramagnet,
where all ![]() particles were distinguishable (by their position in the lattice).
particles were distinguishable (by their position in the lattice).
Consider first the simplest case, of two particles and two energy levels. If the particles are distinguishable,
as in the upper picture below, there are four states, two of which have energy ![]() , and the two-particle
partition function is
, and the two-particle
partition function is
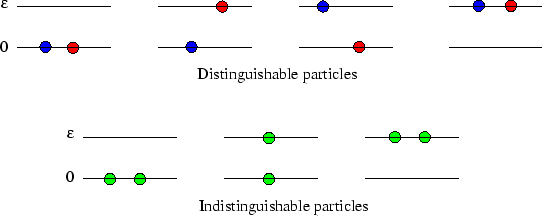
If the particles are indistinguishable, however, there are only three states, as in the lower picture,
and the partition function is
If we use ![]() , we over-count the state in which the particles are in different energy levels. In general there
is no simple expression for the
, we over-count the state in which the particles are in different energy levels. In general there
is no simple expression for the ![]() -particle partition function for indistinguishable particles.
-particle partition function for indistinguishable particles.
However we note that ![]() over-counts the states in which all
over-counts the states in which all ![]() particles are in different energy levels
by exactly
particles are in different energy levels
by exactly ![]() . So if we are in a position where there are many more accessible energy levels (that is, levels
with energy less than a few
. So if we are in a position where there are many more accessible energy levels (that is, levels
with energy less than a few
![]() ) than there are particles, the probability of any two particles being in the
same energy level is small, and almost all states will have all the particles in different levels. Hence a good
approximation is
) than there are particles, the probability of any two particles being in the
same energy level is small, and almost all states will have all the particles in different levels. Hence a good
approximation is
In the ideal gas, we can calculate the number of levels below, say,
![]() , from
, from
![]() with
with
![]() , giving
, giving ![]() . So we see that
. So we see that ![]() is a measure of the number of
states available, and we can use the approximation
is a measure of the number of
states available, and we can use the approximation
![]() provided
provided ![]() (or
(or ![]() ).
This is the classical limit.
).
This is the classical limit.
It is worth noting that, assuming a truly ideal gas which never condenses or solidifies, the Sackur-Tetrode
equation is not valid for indefinitely low temperatures. It must be wrong, because as ![]() ,
, ![]() and
and
![]() . But we know that
. But we know that ![]() as
as ![]() , because all the particles occupy the lowest energy level.
But of course that is exactly the regime in which
, because all the particles occupy the lowest energy level.
But of course that is exactly the regime in which
![]() is no longer valid.
is no longer valid.
For a gas with the density of air at STP,
![]() . We have
. We have ![]() for
for
![]() K, so real gases are essentially always classical.
K, so real gases are essentially always classical.
Note too that
![]() , where
, where ![]() is the wavelength of a particle with energy
is the wavelength of a particle with energy
![]() . This implies that the classical limit holds if the particle separation is large compared
with their wavelength--a reasonable-sounding statement! (See the first tutorial sheet of last semester,
PC210 qu. 1(b-d) ).
. This implies that the classical limit holds if the particle separation is large compared
with their wavelength--a reasonable-sounding statement! (See the first tutorial sheet of last semester,
PC210 qu. 1(b-d) ).
An example of a non-classical gas is the conduction electrons in a metal; they are free to move within the metal and
can be treated as a dense gas (
![]() ), but at room temperature
), but at room temperature
![]() . So the quantum nature
of the electron (specifically the fact that it is a fermion) becomes all important.
. So the quantum nature
of the electron (specifically the fact that it is a fermion) becomes all important.