The change in volume of 1 gram is well approximated by just the volume of the gas phase, since the vapour is
about a thousand times less dense than the liquid:
![]() .
.
So
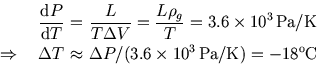
Did you get the right numbers? If not, did you remember to convert the latent heat and the density to refer to the same amount of water (1 kg or 1 g).