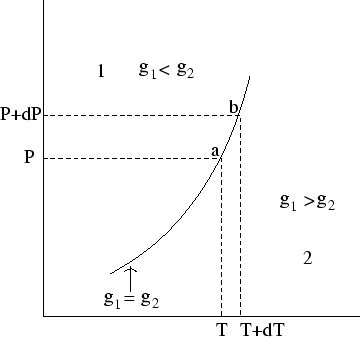
Suppose we know the location of one point on a coexistence line (for instance the melting point at atmospheric pressure). Can we discover other points - for instance, the melting point at a higher or lower pressure? The answer is yes, for very small changes: in fact we can discover the slope of the line.

At both ![]() and
and ![]() the two phases are in equilibrium, so the specific Gibbs free energies of the two phases
at each point are the same:
the two phases are in equilibrium, so the specific Gibbs free energies of the two phases
at each point are the same:
![]() and
and
![]() . So if
. So if ![]() is the difference in
the Gibbs free energy between the two points, it is the same for both phases:
is the difference in
the Gibbs free energy between the two points, it is the same for both phases:
![]() .
But (using small letters
.
But (using small letters ![]() and
and ![]() to denote specific entropy and volume),
to denote specific entropy and volume),
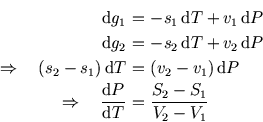
The is called the Clausius-Clapeyron equation, and it relates the slope along the coexistence line with the change in entropy and volume of the substance as it crosses the line, ie changes phase.
This doesn't look very useful, as we can't measure entropy directly. However, using ![]() for an isothermal
process, we can find the change in entropy at a phase transition from the latent heat
for an isothermal
process, we can find the change in entropy at a phase transition from the latent heat ![]() , and so the more useful form
of the equation is
, and so the more useful form
of the equation is