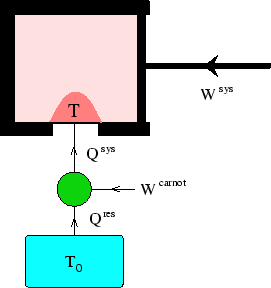

In the diagram, the system is the gas in the piston.
We use a Carnot heat engine/pump to add heat ![]() to the system at a local, varying
temperature
to the system at a local, varying
temperature ![]() . During the process work
. During the process work ![]() is done on the system.
(Both
is done on the system.
(Both ![]() and
and ![]() could be positive or negative.)
The cold reservoir of the Carnot engine is at
could be positive or negative.)
The cold reservoir of the Carnot engine is at ![]() .
.
The Kelvin-Planck statement
of the second law says that at the end of a complete cycle of the system, we cannot
have extracted net work (or else we would have turned heat from the cold reservoir into work).
Looking at the figure to see how the signs of the various works are defined,
that means
![]() . By conservation of energy, and
because the system and engine have returned to their initial states, any net work put in must end up
as heat added to the reservoir:
. By conservation of energy, and
because the system and engine have returned to their initial states, any net work put in must end up
as heat added to the reservoir:
![]() (less than zero because
(less than zero because ![]() is defined as heat extracted).
is defined as heat extracted).
Looking now at the Carnot engine, we see that
if at some point in the cycle we add heat
![]() at temperature
at temperature ![]() , heat
, heat
![]() is extracted from the
reservoir, and
is extracted from the
reservoir, and
Clearly if the system is taken through a reversible cycle, it can be run in reverse
and all quantities will simply change signs. But if ![]() was less than zero originally, it will be
greater for the reversed cycle, implying a net extraction of work and violating the Kelvin-Planck statement.
Thus for a reversible system,
was less than zero originally, it will be
greater for the reversed cycle, implying a net extraction of work and violating the Kelvin-Planck statement.
Thus for a reversible system, ![]() must be exactly zero, and
must be exactly zero, and