Next: Ex. 2
Previous: Heat engine examples
Ex. 1
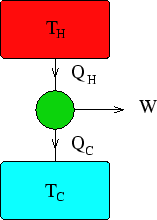
A power station contains a heat engine operating between two heat reservoirs,
one consisting of steam at 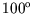 C and the other consisting of water at
C and the other consisting of water at 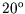 C. What is the
maximum amount of electrical energy which can be produced for every Joule of heat extracted from
the steam?
C. What is the
maximum amount of electrical energy which can be produced for every Joule of heat extracted from
the steam?
The maximum efficiency comes from using a Carnot (reversible) engine, for which
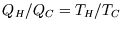 , and so
, and so
With 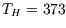 K and
K and 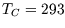 K, for every Joule of heat extracted from the hot reservoir (
K, for every Joule of heat extracted from the hot reservoir (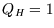 J),
J),
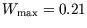 J.
J.





Next: Ex. 2
Previous: Heat engine examples
Judith McGovern
2004-03-17
![]() C and the other consisting of water at
C and the other consisting of water at ![]() C. What is the
maximum amount of electrical energy which can be produced for every Joule of heat extracted from
the steam?
C. What is the
maximum amount of electrical energy which can be produced for every Joule of heat extracted from
the steam?