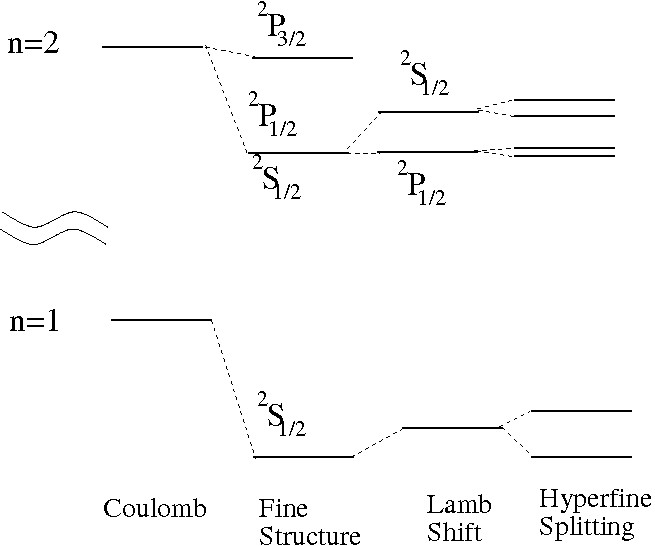
Although the Schrödinger equation with a Coulomb potential reproduces the Bohr model and gives an excellent approximation to the energy levels of hydrogen, the true spectrum was known to be more complicated right from the start. The small deviations are termed “fine structure” and they are of order compared with the ground-state energy (though the equivalent terms for many-electron atoms can be sizable). Hence perturbation theory is an excellent framework in which to consider them.
There are two effects to be considered. One arises from the use of the non-relativistic expression for the kinetic energy, which is only the first term in an expansion of . The first correction term is , and its matrix elements are most easily calculated using the trick of writing it as , where is the usual Hamiltonian with a Coulomb potential. Now in principle we need to be careful here, because is highly degenerate (energies depend only on and not on or ). However we have , and since in this form the operator is spherically symmetric, it can’t link states of different or . So the basis already diagonalises in each subspace of states with the same , and we have no extra work to do here. (We are omitting the superscript on the hydrogenic states, here and below.)
The final result for the kinetic energy effect is
In calculating this the expressions and are useful. Tricks for doing the the radial integrals are explained in Shankar qu. 17.3.4; they are tabulated in section A.3. Details of the algebra for this and the following calculation are given here.
The second correction is the spin-orbit interaction:
In this expression and are the vector operators for orbital and spin angular momentum respectively. The usual (somewhat hand-waving) derivation talks of the electron seeing a magnetic field from the proton which appears to orbit it; the magnetic moment of the electron then prefers to be aligned with this field. This gives an expression which is too large by a factor of 2; an exact derivation requires the Dirac equation.
This time we will run into trouble with the degeneracy of unless we do some work first. The usual trick of writing where tells us that rather than working with eigenstates of and , which would be the basis we’d get with the minimal direct product of the spatial state and a spinor , we want to use eigenstates of and , , instead. (Since for an electron we suppress it in the labelling of the state.) An example of such a state is .
Then
(This expression is only correct for . However there is another separate effect, the Darwin term, which only affects -waves and whose expectation value is just the same as above (with and ), so we can use this for all . The Darwin term can only be understood in the context of the Dirac equation.)
So finally
The degeneracy of all states with the same has been broken. States of are still degenerate, a result that persists to all orders in the Dirac equation (where in any case orbital angular momentum is no longer a good quantum number.) So the eight states are split by eV, with the state lying higher that the degerate and states.
Two other effects should be mentioned here. One is the hyperfine splitting. The proton has a magnetic moment, and the energy of the atom depends on whether the electon spin is aligned with it or not— more precisely, whether the total spin of the electon and proton is or . The anti-aligned case has lower energy (since the charges are opposite), and the splitting for the state is eV. (It is around a factor of 10 smaller for any of the states.) Transitions between the two hyperfine states of hydrogen give rise to the 21 cm microwave radiation which is a signal of cold hydrogen gas in the galaxy and beyond.
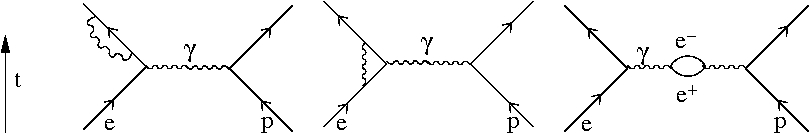
The final effect is called the Lamb shift. It cannot be accounted for in quantum mechanics, but only in quantum field theory.

The diagrams above show corrections to the simple Coulomb force which would be represented by the exchange of a single photon between the proton and the electron. The most notable effect on the spectrum of hydrogen is to lift the remaining degeneracy between the and states, so that the latter is higher by eV.
Below the various corrections to the energy levels of hydrogen are shown schematically. The gap between the and shells is supressed, and the Lamb and hyperfine shifts are exaggerated in comparison with the fine-structure. The effect of the last two on the level is not shown.