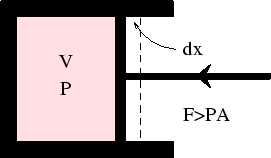
First, we consider the work done in compressing a fluid (hydrodynamic system).

In any real case, compression requires the applied force to be greater than the internal pressure times the area
of the piston: ![]() . The work done in moving the piston though
. The work done in moving the piston though ![]() is
is
If there is no friction, and the compression is done extremely slowly, the applied force will only be
barely greater than ![]() . In that case the process is reversible, and the inequality will become
an equality:
. In that case the process is reversible, and the inequality will become
an equality:
![$\mbox{\large\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle
{}\raise0.44ex\hbox{\bf\symbol{'040}}\llap{d}W^{\rm rev}= - P {\rm d}V$ }}$](img54.gif)
Similarly to reversibly stretch a wire of tension ![]() (that's a capital gamma) by
(that's a capital gamma) by ![]() requires
requires
![$\mbox{\large\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle
{}\raise0.44ex\hbox{\bf\symbol{'040}}\llap{d}W^{\rm rev}= \Gamma {\rm d}l$ }}$](img57.gif)
and to increase the area of a film of surface tension ![]() by
by ![]() requires
requires
![$\mbox{\large\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle
{}\raise0.44ex\hbox{\bf\symbol{'040}}\llap{d}W^{\rm rev}= \gamma {\rm d}A$ }}$](img60.gif)
Lastly, to reversibly increase the magnetic field ![]() imposed upon a paramagnetic sample requires
imposed upon a paramagnetic sample requires
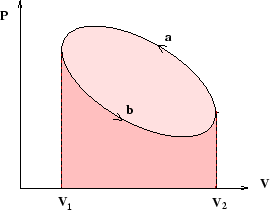
If reversible processes are represented by lines on a plot of pressure against volume (or tension against length, or...), then the magnitude of the work done is equal to the area under the line. Cycles are closed loops as in the picture above, and the magnitude of the work done is equal to the area of the loop (that is, the difference between the areas under the two lines).
Test yourself with this example. More can be found on the tutorial sheet.
References