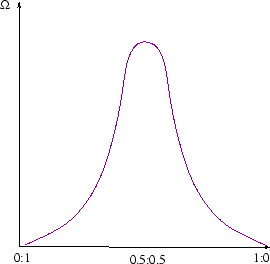
Suppose a system has an extra degree of freedom which isn't specified by fixing the volume and internal energy. For a mixture of two ideal gases, it could be the degree to which they are fully mixed-for example ratio of the concentrations of one species on either side of the box. If the gases started out separated by a partition the concentrations would start at 0:1; the classical law of increase of entropy tells us they will evolve till the ratio reaches 0.5:0.5, and not change thereafter. (See here for a calculation of the entropy change). At the classical level, we don't understand this yet. It is just a law of nature, deduced ultimately from observation.
Statistical physics can explain the spontaneous increase of entropy. There are many more microstates corresponding to the equilibrium configuration (fully mixed) than the non-equilibrium configurations (not fully mixed). The number of microstates as a function of mixing looks something like this, but really much sharper:

What has this to do with entropy? Classically, the system is evolving from a macrostate of lower entropy to one of higher entropy. Statistically, it is evolving from less probable to more probable macrostates, that is from macrostates corresponding to smaller numbers of microstates to those corresponding to larger numbers of microstates.
So does the number of microstates, ![]() , equal the entropy? No, because if we double the size of a system,
we have
, equal the entropy? No, because if we double the size of a system,
we have ![]() , not
, not ![]() , microstates (think of the number of ways of choosing the microstate of
each half independently). So
, microstates (think of the number of ways of choosing the microstate of
each half independently). So ![]() isn't extensive. But
isn't extensive. But ![]() is. So if we make the connection
is. So if we make the connection
In principle, if the increase of entropy is just a probabilistic thing, it might sometimes decrease. However we will see that for macroscopic systems the odds are so overwhelmingly against an observable decrease that we might as well say it will never happen.
What is
![]() , Boltzmann's constant? It must be a constant with dimensions of entropy, Joules/Kelvin, and it turns
out that the correct numerical correspondence is given by the gas constant
, Boltzmann's constant? It must be a constant with dimensions of entropy, Joules/Kelvin, and it turns
out that the correct numerical correspondence is given by the gas constant ![]() divided by Avogadro's number:
divided by Avogadro's number:
Find a simple example of these ideas here.
References